By: Robyn Woidtke, MSN-Ed, RN, RPSGT, CCSH, FAAST
CAVEAT: I AM NOT A REIMBURSEMENT; LEGAL OR CMS EXPERT
In 2021, partially in response to the Covid pandemic, the Centers for Medicare and Medicaid (CMS) provided an update for the use of remote and therapeutic patient monitoring. I was pleased to note that Telehealth.HHS.gov actually mentioned sleep apnea on their information page. The sleep industry has been keeping pace as the digital world increasingly intersects with patients and caregivers. Several platforms are emerging in this space that provides the sleep medicine team access to data and patient outcomes.
What is the difference between RPM and RTM? [1]
According to CMS, remote patient monitoring (AKA physiological monitoring) is defined as the use of digital technologies to collect health data from patients in one location and electronically transmit that information securely to providers in a different location (data can include vital signs, weight, blood pressure, blood sugar, pacemaker information, etc.)[2]
An article by Foley and Lardner LLC[3]states, “RTM is designed for the management of patients using medical devices that collect non-physiological data. Data around indicators such as therapy/medication adherence, therapy/medication response, and pain level can be collected and billed under the new RTM codes…CMS recognizes “therapeutic” data—not just “physiologic” data—as an important category of patient information that can be assessed remotely.”
There are different codes for RPM and RTM. Codes can be used for real-time and asynchronous patient interaction. [4]
According to several resources, likely new codes are coming in 2023, which have addressed one of the most significant issues with providing RPM/RTM services. This clause was the requirement for direct supervision. The proposed wording includes “general” supervision.
I thought this sentence in the Federal Register document enlightening “We (CMS) seek information related to the types of data collected using RTM devices, how the data that are collected solve specific health conditions and what those health conditions are, the costs associated with RTM devices that are available to collect RTM data, how long the typical episode of care by condition type might last, and the potential number of beneficiaries for whom an RTM device might be used by the health condition type”, It would be advantageous for the sleep world to become engaged, if not already in these discussions.”
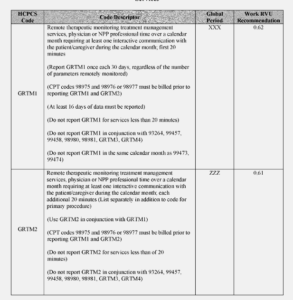
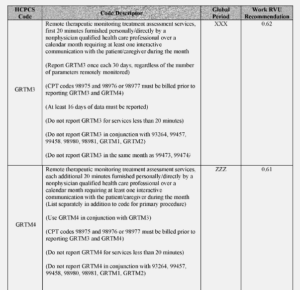
Another section of note from the Federal Register, which may have direct applicability to the sleep world “ for CY 2023, as a means of increasing beneficiary access to RTM services, as well as more clearly defining the services of RTM for qualified nonphysician healthcare practitioners whose Medicare benefit category does not include services provided incident to their own services, we are proposing two codes that would expressly facilitate RTM services furnished by qualified nonphysician healthcare professionals who cannot bill under Medicare Part B for services furnished incident to their professional services (Codes GRTM3 and GRTM4)
And finally, “CPT codes 98980 and 98981 must be furnished under the direct supervision of the billing practitioner, which imposes burden on physicians and NPPs who are delivering services to other patients. Thus, for CY 2023, we are proposing to create two HCPCS G codes, one base code and one add-on code, that include clinical labor activities (that is, incident to services such as communicating with the patient, resolving technology concerns, reviewing data, updating and modifying care plans, and addressing lack of patient improvement) that can be furnished by auxiliary personnel under general supervision.”
It would seem that this new wording can positively impact patients regarding sleep health outcomes and how care may be delivered in the future. (See code definitions below)
- GRTM3 (Remote therapeutic monitoring treatment assessment services, first 20 minutes furnished personally/directly by a nonphysician qualified health care professional over a calendar month requiring at least one interactive communication with the patient/caregiver during the month).
- GRTM4 (Remote therapeutic monitoring treatment assessment services, additional 20 minutes furnished personally/directly by a nonphysician qualified health care professional over a calendar month requiring at least one interactive communication with the patient/caregiver during the calendar month (List separately in addition to code for primary procedure)).
In the FDA world, there are different categories of types of medical devices, one of which includes software as a medical device (SaMD). In view of the fact that RTM still requires a medical device designation, a platform that can capture patient data input by the patient may qualify under this category. Another category is medical device data systems. Some, but not all are considered a medical device. It is important when choosing a system to aid in RPM/RTM, that you assess the requirements and if the platform meets what is needed. Thus, the difference is that data can be collected in other ways, not just through a medical device (i.e., PAP). However, it is good to know that these codes specifically mention respiratory monitoring. And lastly, don’t forget the reasonable and necessary language from CMS.
In summary, practices, organizations and sleep practioners should be aware of these potential opportunities in remote therapeutic monitoring to enhance patient care. Care when selecting or creating a platform should take into account all of the CMS requirements.[5]
See the RTM Code table below from the Federal Register.
[1] https://www.federalregister.gov/documents/2022/07/29/2022-14562/medicare-and-medicaid-programs-cy-2023-payment-policies-under-the-physician-fee-schedule-and-other
[2] https://www.cms.gov/files/document/telehealth-toolkit-providers.pdf
[3] https://www.foley.com/en/insights/publications/2021/11/2022-remote-therapeutic-monitoring-cms-final-rule
[4] https://healthsnap.io/2023-medicare-physician-fee-schedule-proposed-changes-what-you-need-to-know/
[5] https://www.cms.gov/files/document/r11118cp.pdf